Which of the Following Is a Lewis Acid
Lewis suggested another way of looking at the reaction between H and OH ions. Studies from the Drover group began with the coordination chemistry of NiP 2 B Cy 4 2 and investigations into the influence of a Lewis-acidic secondary coordination sphere on.
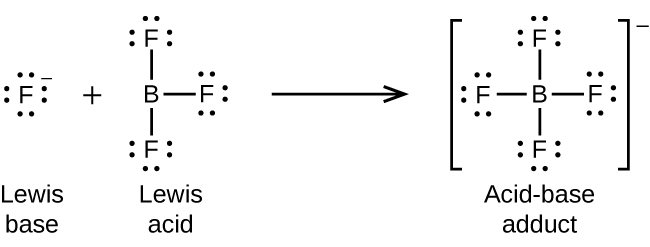
15 2 Lewis Acids And Bases Chemistry
Most likely to be useful to students in year long rather than survey courses 3.

. C 6 H 6 CH 3 C 5 H 5 CHCH 3 in which the methyl carbocation has formed a sigma bond with one carbon atom of the benzene ring by accepting one of the 3 pairs of pi electrons of the ring in a. A SbH 3 b XeF 2 c Se 8 a cyclic molecule with a ring of eight Se atoms Methanol H 3 COH is used as the fuel in some race cars. Since boron is electron deficient it does not have a valence shell electron octet the reagent itself is a Lewis acid and can bond to the pi-electrons of a double bond by displacement of the ether moiety from the solvated monomer.
The prime importance of this work is that we incorporate the Lewis acidity in zeolite Y by simple ion-exchange procedure and then systematically correlate the structure of these Lewis acidic species to their concentration and catalytic performance which is a valuable development towards understanding the nature and role of Lewis acid sites in zeolite catalysis. The difference between the two is that muriatic acid is a strong acid and vinegar is a weak acid. The problems have been color-coded to indicate whether they are.
There are three single bonds in the molecule. Therefore a Lewis acid is any substance such as the H ion that can accept a pair of nonbonding. As shown in the following equation this bonding might generate a dipolar intermediate consisting of a negatively-charged boron and a carbocation.
Most likely to be useful only to students in courses for chemistry. Lewiss definition of Base is that any chemical compound that has the ability to donate lone pairs to other chemical species can act as a lewis base. This conjugate BrønstedLewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University and has been used to stabilize carbocations and hypercoordinated.
Sr Se -. Lewis-acidic secondary coordination spheres offer an opportunity for engagement with Lewis bases providing entry points into macromolecular complexes having Lewis acidbase interactions. Write Lewis structures for the following molecules or ions.
Both methanol and ethanol produce CO 2 and H 2 O when they burn. We know that in NH3 NitrogenN has 5 electrons in its valence shell with the configuration of 1s2 2s2 sp3 and Hydrogen has only 1. Hence the shape of the formic acid can easily be predicted by the following table.
There are no charges at any atom. One hydrogen atom is joint to phosphorous atom and remaining. Acting as a Lewis Acid NH3 NH2- H.
In this step the Lewis acid AlCl 3 extracts the Lewis base Cl- from its adduct with the Lewis acid CH 3. BF 3 is a Lewis acid. Ethanol C 2 H 5 OH is used extensively as motor fuel in Brazil.
HClaq H 2 Ol H 3 O aq Cl-aq. Lewis structure of phosphoric acid contains -1 charge on one oxygen atom and 1 charge on phosphorous atom. What is the Lewis dot structure for Sr Se SrSe Write the Lewis dot symbols of the reactants and products in the following reactions.
In a 6 M solution of hydrochloric acid 99996 of the HCl molecules react with water to form H 3 O and Cl-ions. Why NH3 acts as a Lewis Base. In VSEPR theory the double bond is treated as one bond pair for the prediction of the shape of the molecule.
The products are the acid CH 3 and a new adduct AlCl 4 -. According to the Lewis structure of formic acid the carbon atom is a central atom and it has three bond pairs without any lone pair of electrons. Muriatic acid is strong because it is very good at transferring an H ion to a water molecule.
First balance equations a. What is correct about from following statements about the lewis structure of phosphoric acid. Write the chemical equations for these combustion.
In the Lewis theory of acid-base reactions bases donate pairs of electrons and acids accept electrons pairs. Magic acid FSO 3 HSbF 5 is a superacid consisting of a mixture most commonly in a 11 molar ratio of fluorosulfuric acid HSO 3 F and antimony pentafluoride SbF 5. The following problems are meant to be useful study tools for students involved in most undergraduate organic chemistry courses.
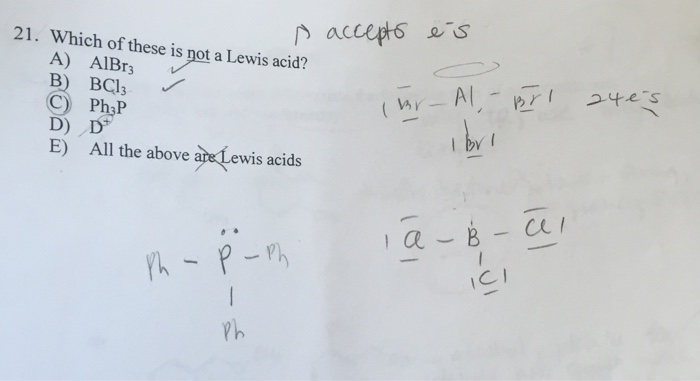
Solved Which Of These Is Not A Lewis Acid Albr 3 Bcl 3 All Chegg Com

Which Of Thef Following Is Not Lewis Acid Youtube
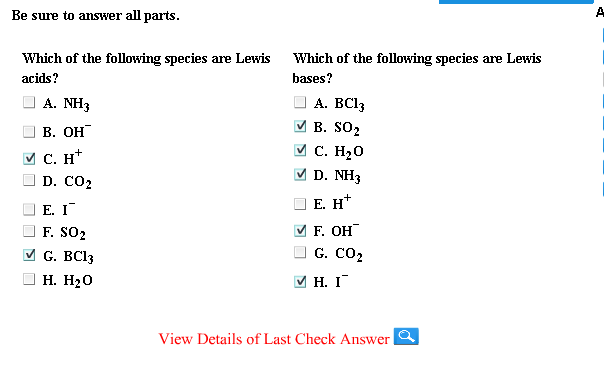
Solved Which Of The Following Species Are Lewis Acids Nh3 Chegg Com
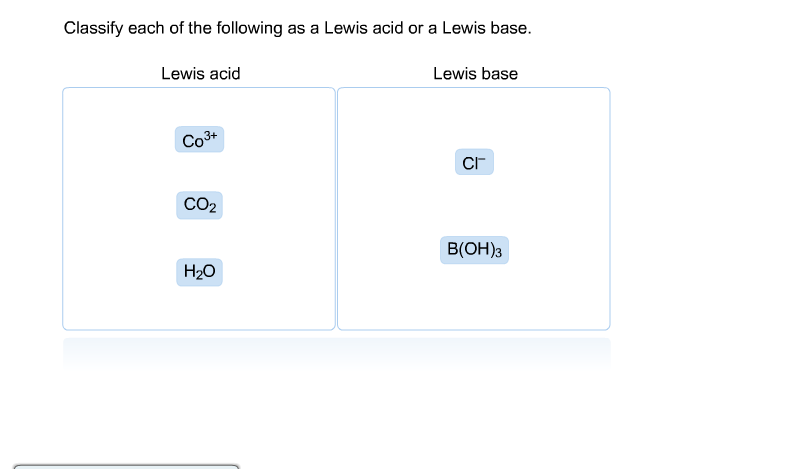
Solved Of The Following As A Lewis Acid Or A Lewis Base Chegg Com

Solved Question 16 Which Of The Following Is A Lewis Acid Chegg Com

Which Of The Following Is A Lewis Acid But Not A Bronsted Acid Youtube

Which Of The Following Is Not Lewis Acid

Lewis Acids And Bases Chemistry Steps
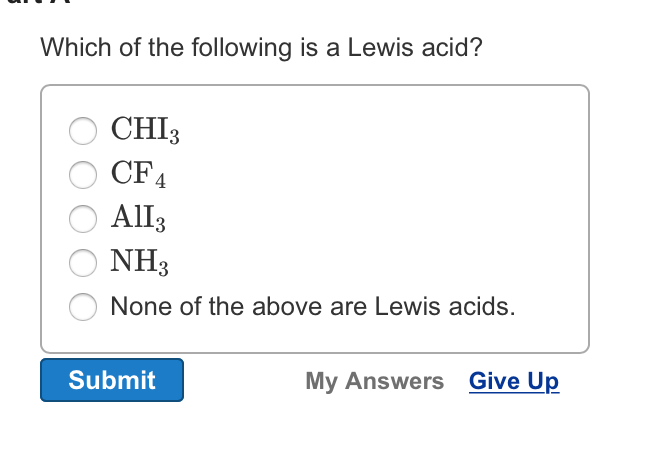
Solved Which Of The Following Is A Lewis Acid Chi 3 Cf 4 Chegg Com

Solved Which Of The Following Are Lewis Acids And Which Of Chegg Com

Solved 6 Which Of The Following Is A Lewis Acid A All3 Chegg Com
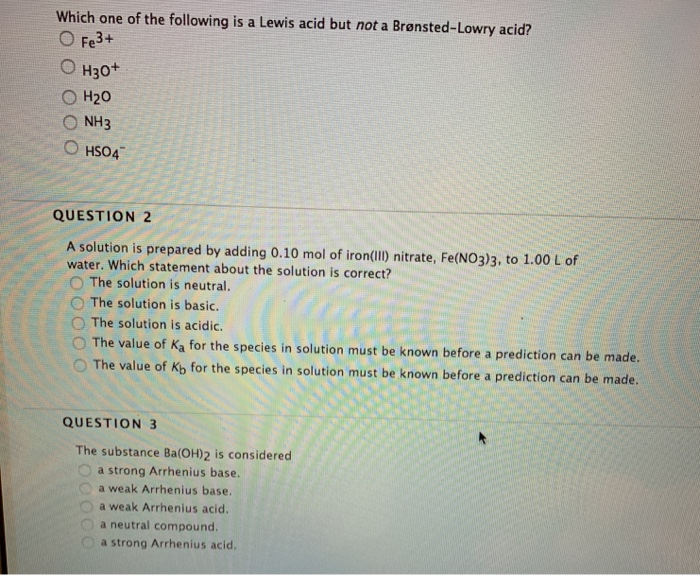
Solved Which One Of The Following Is A Lewis Acid But Not A Chegg Com

Which Of The Following Is A Lewis Acid Youtube
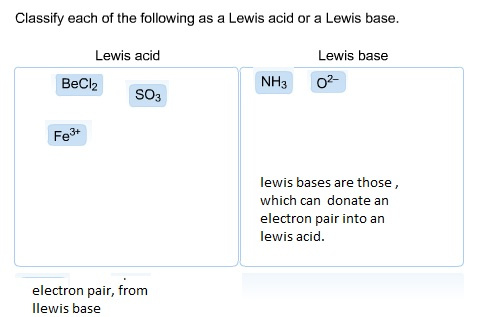
Classify Each Of The Following As A Lewis Acid Or A Lewis Base Explain How To Determine Lewis Acids And Bases Home Work Help Learn Cbse Forum
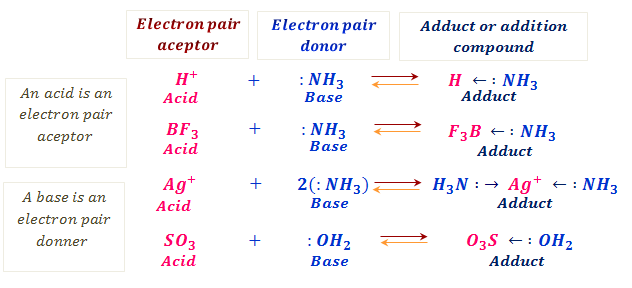
Example Of Lewis Acid Base Each Of The Concepts Had Its Own By Chemistry Topics Inorganic Chemistry Topics Medium

Lewis Acids And Bases Definition Properties Examples Reactions Uses Applications Of Lewis Acids And Bases
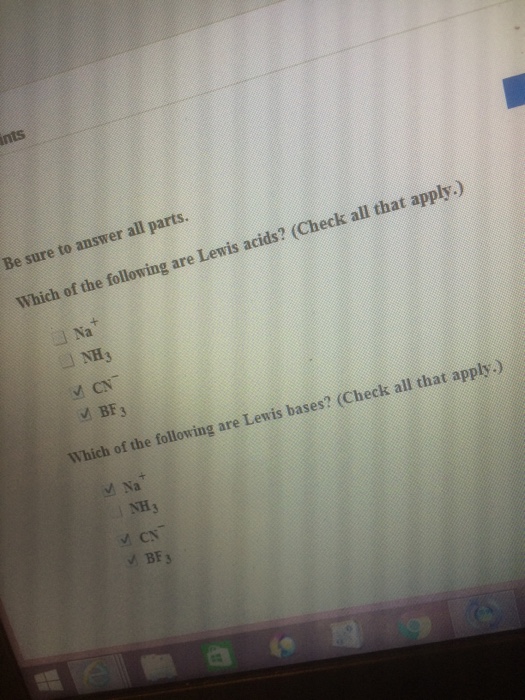
Solved Which Of The Following Are Lewis Acids Which Of The Chegg Com
Comments
Post a Comment